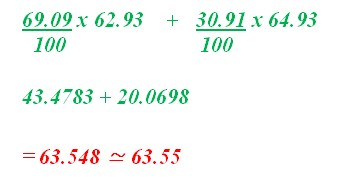Question
The table below shows the relative atomic masses and the percentage abundance of the isotopes L1 and L2 of element L.
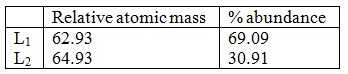
Calculate the relative atomic mass of element K.
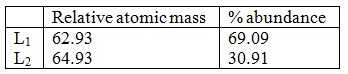
Calculate the relative atomic mass of element K.
Answer